9.2 – Signaling Pathways, Hormones and Endocrine System
 |
9.2. Describe general cell signaling strategies and explain types of hormones and their signaling pathways in endocrine communication. |
Chemical signaling
There are two kinds of communication in the world of living cells. Communication between cells is called intercellular signaling, and communication within a cell is called intracellular signaling. An easy way to remember the distinction is by understanding the Latin origin of the prefixes: inter- means “between” (for example, intersecting lines are those that cross each other) and intra- means “inside” (like intravenous).
Chemical signals are released by signaling cells in the form of small, usually volatile or soluble molecules called ligands. A ligand is a molecule that binds another specific molecule, in some cases, delivering a signal in the process. Ligands can thus be thought of as signaling molecules. Ligands interact with proteins in target cells, which are cells that are affected by chemical signals; these proteins are also called receptors. Ligands and receptors exist in several varieties; however, a specific ligand will have a specific receptor that typically binds only that ligand.
Forms of Signaling
There are four categories of chemical signaling found in multicellular organisms: paracrine signaling, endocrine signaling, autocrine signaling, and direct signaling across gap junctions (Figure 9.2). The main difference between the different categories of signaling is the distance that the signal travels through the organism to reach the target cell. Not all cells are affected by the same signals.
In chemical signaling, a cell may target itself (autocrine signaling), a cell connected by gap junctions, a nearby cell (paracrine signaling), or a distant cell (endocrine signaling). Paracrine signaling acts on nearby cells, endocrine signaling uses the circulatory system to transport ligands, and autocrine signaling acts on the signaling cell. Signaling via gap junctions involves signaling molecules moving directly between adjacent cells.
Paracrine signaling
Signals that act locally between cells that are close together are called paracrine signals. Paracrine signals move by diffusion through the extracellular matrix. These types of signals usually elicit quick responses that last only a short amount of time. In order to keep the response localized, paracrine ligand molecules are normally quickly degraded by enzymes or removed by neighboring cells. Removing the signals will reestablish the concentration gradient for the signal, allowing them to quickly diffuse through the intracellular space if released again.
Endocrine signaling
Signals from distant cells are called endocrine signals, and they originate from endocrine cells. (In the body, many endocrine cells are located in endocrine glands, such as the thyroid gland, the hypothalamus, and the pituitary gland.) These types of signals usually produce a slower response but have a longer-lasting effect. The ligands released in endocrine signaling are called hormones, signaling molecules that are produced in one part of the body but affect other body regions some distance away.
Hormones travel the large distances between endocrine cells and their target cells via the bloodstream, which is a relatively slow way to move throughout the body. Because of their form of transport, hormones get diluted and are present in low concentrations when they act on their target cells. This is different from paracrine signaling, in which local concentrations of ligands can be very high.
Autocrine signaling
Autocrine signals are produced by signaling cells that can also bind to the ligand that is released. This means the signaling cell and the target cell can be the same or a similar cell (the prefix auto- means self, a reminder that the signaling cell sends a signal to itself). This type of signaling often occurs during the early development of an organism to ensure that cells develop into the correct tissues and take on the proper function. Autocrine signaling also regulates pain sensation and inflammatory responses. Further, if a cell is infected with a virus, the cell can signal itself to undergo programmed cell death, killing the virus in the process. In some cases, neighboring cells of the same type are also influenced by the released ligand. In embryological development, this process of stimulating a group of neighboring cells may help to direct the differentiation of identical cells into the same cell type, thus ensuring the proper developmental outcome.
Direct signaling across gap junctions
Gap junctions in animals and plasmodesmata in plants are connections between the plasma membranes of neighboring cells. These water-filled channels allow small signaling molecules, called intracellular mediators, to diffuse between the two cells. Small molecules, such as calcium ions (Ca2+), are able to move between cells, but large molecules like proteins and DNA cannot fit through the channels. The specificity of the channels ensures that the cells remain independent but can quickly and easily transmit signals. The transfer of signaling molecules communicates the current state of the cell that is directly next to the target cell; this allows a group of cells to coordinate their response to a signal that only one of them may have received. In plants, plasmodesmata are ubiquitous, making the entire plant into a giant, communication network.
Types of receptors
Receptors are protein molecules in the target cell or on its surface that bind ligand. There are two types of receptors, internal receptors and cell-surface receptors.
Internal receptors
Internal receptors, also known as intracellular or cytoplasmic receptors, are found in the cytoplasm of the cell and respond to hydrophobic ligand molecules that are able to travel across the plasma membrane. Once inside the cell, many of these molecules bind to proteins that act as regulators of mRNA synthesis (transcription) to mediate gene expression. Gene expression is the cellular process of transforming the information in a cell’s DNA into a sequence of amino acids, which ultimately forms a protein. When the ligand binds to the internal receptor, a conformational change is triggered that exposes a DNA-binding site on the protein. The ligand-receptor complex moves into the nucleus, then binds to specific regulatory regions of the chromosomal DNA and promotes the initiation of transcription (Figure 9.3). Transcription is the process of copying the information in a cells DNA into a special form of RNA called messenger RNA (mRNA); the cell uses information in the mRNA (which moves out into the cytoplasm and associates with ribosomes) to link specific amino acids in the correct order, producing a protein. Internal receptors can directly influence gene expression without having to pass the signal on to other receptors or messengers.
Hydrophobic signaling molecules typically diffuse across the plasma membrane and interact with intracellular receptors in the cytoplasm. Many intracellular receptors are transcription factors that interact with DNA in the nucleus and regulate gene expression.
Cell-surface receptors
Cell-surface receptors, also known as transmembrane receptors, are cell surface, membrane-anchored (integral) proteins that bind to external ligand molecules. This type of receptor spans the plasma membrane and performs signal transduction, in which an extracellular signal is converted into an intercellular signal. Ligands that interact with cell-surface receptors do not have to enter the cell that they affect. Cell-surface receptors are also called cell-specific proteins or markers because they are specific to individual cell types.
Because cell-surface receptor proteins are fundamental to normal cell functioning, it should come as no surprise that a malfunction in any one of these proteins could have severe consequences. Errors in the protein structures of certain receptor molecules have been shown to play a role in hypertension (high blood pressure), asthma, heart disease, and cancer.
Each cell-surface receptor has three main components: an external ligand-binding domain, a hydrophobic membrane-spanning region, and an intracellular domain inside the cell. The ligand-binding domain is also called the extracellular domain. The size and extent of each of these domains vary widely, depending on the type of receptor. Cell-surface receptors are involved in most of the signaling in multicellular organisms. There are three general categories of cell-surface receptors: ion channel-linked receptors, G-protein-linked receptors, and enzyme-linked receptors.
Ion channel-linked receptors bind a ligand and open a channel through the membrane that allows specific ions to pass through. To form a channel, this type of cell-surface receptor has an extensive membrane-spanning region. In order to interact with the phospholipid fatty acid tails that form the center of the plasma membrane, many of the amino acids in the membrane-spanning region are hydrophobic in nature. Conversely, the amino acids that line the inside of the channel are hydrophilic to allow for the passage of water or ions. When a ligand binds to the extracellular region of the channel, there is a conformational change in the proteins structure that allows ions such as sodium, calcium, magnesium, and hydrogen to pass through (Figure 9.4)
G-protein-linked receptors bind a ligand and activate a membrane protein called a G-protein. The activated G-protein then interacts with either an ion channel or an enzyme in the membrane (Figure 9.5). All G-protein-linked receptors have seven transmembrane domains, but each receptor has its own specific extracellular domain and G-protein-binding site.
Cell signaling using G-protein-linked receptors occurs as a cyclic series of events. Before the ligand binds, the inactive G-protein can bind to a newly revealed site on the receptor specific for its binding. Once the G-protein binds to the receptor, the resultant shape change activates the G-protein, which releases GDP and picks up GTP. The subunits of the G-protein then split into the α subunit and the βγ subunit. One or both of these G-protein fragments may be able to activate other proteins as a result. After a while, the GTP on the active α subunit of the G-protein is hydrolyzed to GDP and the βγ subunit is deactivated. The subunits reassociate to form the inactive G-protein and the cycle begins anew.

Figure 9.5. Heterotrimeric G proteins have three subunits: α, β, and γ. When a signaling molecule binds to a G-protein-coupled receptor in the plasma membrane, a GDP molecule associated with the α subunit is exchanged for GTP. The β and γ subunits dissociate from the α subunit, and a cellular response is triggered either by the α subunit or the dissociated βγ pair. Hydrolysis of GTP to GDP terminates the signal.
G-protein-linked receptors have been extensively studied and much has been learned about their roles in maintaining health. Bacteria that are pathogenic to humans can release poisons that interrupt specific G-protein-linked receptor function, leading to illnesses such as pertussis, botulism, and cholera. In cholera (Figure 9.6), for example, the water-borne bacterium Vibrio cholerae produces a toxin, choleragen, that binds to cells lining the small intestine. The toxin then enters these intestinal cells, where it modifies a G-protein that controls the opening of a chloride channel and causes it to remain continuously active, resulting in large losses of fluids from the body and potentially fatal dehydration as a result.

Figure 9.6. Transmitted primarily through contaminated drinking water, cholera is a major cause of death in the developing world and in areas where natural disasters interrupt the availability of clean water. The cholera bacterium, Vibrio cholerae, creates a toxin that modifies G-protein-mediated cell signaling pathways in the intestines. Modern sanitation eliminates the threat of cholera outbreaks, such as the one that swept through New York City in 1866. This poster from that era shows how, at that time, the way that the disease was transmitted was not understood. (credit: New York City Sanitary Commission)
Enzyme-linked receptors are cell-surface receptors with intracellular domains that are associated with an enzyme. In some cases, the intracellular domain of the receptor itself is an enzyme. Other enzyme-linked receptors have a small intracellular domain that interacts directly with an enzyme. The enzyme-linked receptors normally have large extracellular and intracellular domains, but the membrane-spanning region consists of a single alpha-helical region of the peptide strand. When a ligand binds to the extracellular domain, a signal is transferred through the membrane, activating the enzyme. Activation of the enzyme sets off a chain of events within the cell that eventually leads to a response. One example of this type of enzyme-linked receptor is the tyrosine kinase receptor (Figure 9.7). A kinase is an enzyme that transfers phosphate groups from ATP to another protein. The tyrosine kinase receptor transfers phosphate groups to tyrosine molecules (tyrosine residues). First, signaling molecules bind to the extracellular domain of two nearby tyrosine kinase receptors. The two neighboring receptors then bond together, or dimerize. Phosphates are then added to tyrosine residues on the intracellular domain of the receptors (phosphorylation). The phosphorylated residues can then transmit the signal to the next messenger within the cytoplasm.

Figure 9.7. A receptor tyrosine kinase is an enzyme-linked receptor with a single transmembrane region and extracellular and intracellular domains. Binding of a signaling molecule to the extracellular domain causes the receptor to dimerize. Tyrosine residues on the intracellular domain are then autophosphorylated, triggering a downstream cellular response. The signal is terminated by a phosphatase that removes the phosphates from the phosphotyrosine residues.
Signaling molecules
Produced by signaling cells and the subsequent binding to receptors in target cells, ligands act as chemical signals that travel to the target cells to coordinate responses. The types of molecules that serve as ligands are incredibly varied and range from small proteins to small ions like calcium (Ca2+).
Small hydrophobic ligands
Small hydrophobic ligands can directly diffuse through the plasma membrane and interact with internal receptors. Important members of this class of ligands are the steroid hormones. Steroids are lipids that have a hydrocarbon skeleton with four fused rings; different steroids have different functional groups attached to the carbon skeleton. Steroid hormones include the female sex hormone, estradiol, which is a type of estrogen; the male sex hormone, testosterone; and cholesterol, which is an important structural component of biological membranes and a precursor of steroid hormones (Figure 9.8). Other hydrophobic hormones include thyroid hormones and vitamin D. In order to be soluble in blood, hydrophobic ligands must bind to carrier proteins while they are being transported through the bloodstream.

Figure 9.8. Steroid hormones have similar chemical structures to their precursor, cholesterol. Because these molecules are small and hydrophobic, they can diffuse directly across the plasma membrane into the cell, where they interact with internal receptors.
Water-soluble ligands
Water-soluble ligands are polar and therefore cannot pass through the plasma membrane unaided; sometimes, they are too large to pass through the membrane at all. Instead, most water-soluble ligands bind to the extracellular domain of cell-surface receptors. This group of ligands is quite diverse and includes small molecules, peptides, and proteins.
Other ligands
Nitric oxide (NO) is a gas that also acts as a ligand. It is able to diffuse directly across the plasma membrane, and one of its roles is to interact with receptors in smooth muscle and induce relaxation of the tissue. NO has a very short half-life and therefore only functions over short distances. Nitroglycerin, a treatment for heart disease, acts by triggering the release of NO, which causes blood vessels to dilate (expand), thus restoring blood flow to the heart. NO has become better known recently because the pathway that it affects is targeted by prescription medications for erectile dysfunction, such as Viagra (erection involves dilated blood vessels).
Hormones
Hormones mediate changes in target cells by binding to specific hormone receptors. In this way, even though hormones circulate throughout the body and come into contact with many different cell types, they only affect cells that possess the necessary receptors. Receptors for a specific hormone may be found on many different cells or may be limited to a small number of specialized cells. For example, thyroid hormones act on many different tissue types, stimulating metabolic activity throughout the body. Cells can have many receptors for the same hormone but often also possess receptors for different types of hormones. The number of receptors that respond to a hormone determines the cell’s sensitivity to that hormone and the resulting cellular response. Additionally, the number of receptors that respond to a hormone can change over time, resulting in increased or decreased cell sensitivity. In up-regulation, the number of receptors increases in response to rising hormone levels, making the cell more sensitive to the hormone and allowing for more cellular activity. When the number of receptors decreases in response to rising hormone levels, called down-regulation, cellular activity is reduced.
Receptor binding alters the cellular activity and results in an increase or decrease in normal body processes. Depending on the location of the protein receptor on the target cell and the chemical structure of the hormone, hormones can mediate changes directly by binding to intracellular hormone receptors and modulating gene transcription, or indirectly by binding to cell surface receptors and stimulating signaling pathways.
Intracellular hormone receptors
Lipid-derived (soluble) hormones such as steroid hormones diffuse across the membranes of the endocrine cell. Once outside the cell, they bind to transport proteins that keep them soluble in the bloodstream. At the target cell, the hormones are released from the carrier protein and diffuse across the lipid bilayer of the plasma membrane of cells. The steroid hormones pass through the plasma membrane of a target cell and adhere to intracellular receptors residing in the cytoplasm or in the nucleus. The cell signaling pathways induced by the steroid hormones regulate specific genes on the cell’s DNA. The hormones and receptor complex act as transcription regulators by increasing or decreasing the synthesis of mRNA molecules of specific genes. This, in turn, determines the amount of corresponding protein that is synthesized by altering gene expression. This protein can be used either to change the structure of the cell or to produce enzymes that catalyze chemical reactions. In this way, the steroid hormone regulates specific cell processes as illustrated in Figure 9.9.
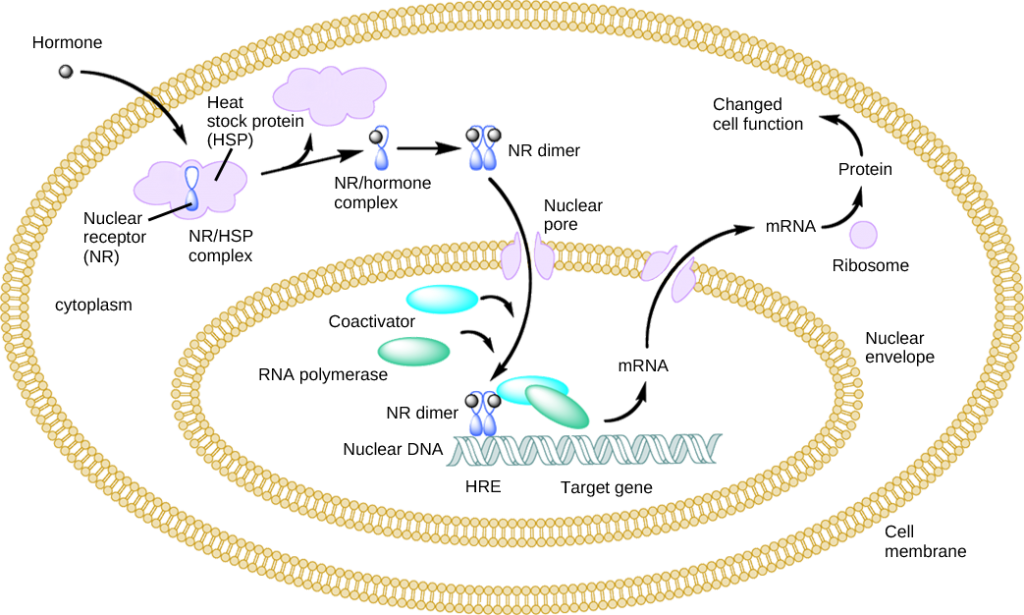
Figure 9.9. Heat shock proteins (HSP) are so named because they help refold misfolded proteins. In response to increased temperature (a “heat shock”), heat shock proteins are activated by release from the NR/HSP complex. At the same time, transcription of HSP genes is activated. Why do you think the cell responds to a heat shock by increasing the activity of proteins that help refold misfolded proteins?
Other lipid-soluble hormones that are not steroid hormones, such as vitamin D and thyroxine, have receptors located in the nucleus. The hormones diffuse across both the plasma membrane and the nuclear envelope, then bind to receptors in the nucleus. The hormone-receptor complex stimulates transcription of specific genes.
Plasma membrane hormone receptors
Amino acid derived hormones and polypeptide hormones are not lipid-derived (lipid-soluble) and therefore cannot diffuse through the plasma membrane of cells. Lipid insoluble hormones bind to receptors on the outer surface of the plasma membrane, via plasma membrane hormone receptors. Unlike steroid hormones, lipid-insoluble hormones do not directly affect the target cell because they cannot enter the cell and act directly on DNA. Binding of these hormones to a cell surface receptor results in the activation of a signaling pathway; this triggers intracellular activity and carries out the specific effects associated with the hormone. In this way, nothing passes through the cell membrane; the hormone that binds at the surface remains at the surface of the cell while the intracellular product remains inside the cell. The hormone that initiates the signaling pathway is called a first messenger, which activates a second messenger in the cytoplasm, as illustrated in Figure 9.6.
One very important second messenger is cyclic AMP (cAMP). When a hormone binds to its membrane receptor, a G-protein that is associated with the receptor is activated; G-proteins are proteins separate from receptors that are found in the cell membrane (Figure 9.5). When a hormone is not bound to the receptor, the G-protein is inactive and is bound to guanosine diphosphate or GDP. When a hormone binds to the receptor, the G-protein is activated by binding guanosine triphosphate, or GTP, in place of GDP. After binding, GTP is hydrolysed by the G-protein into GDP and becomes inactive. The activated G-protein, in turn, activates a membrane-bound enzyme called adenylyl cyclase. Adenylyl cyclase catalyzes the conversion of ATP to cAMP. cAMP, in turn, activates a group of proteins called protein kinases, which transfer a phosphate group from ATP to a substrate molecule in a process called phosphorylation. The phosphorylation of a substrate molecule changes its structural orientation, thereby activating it. These activated molecules can then mediate changes in cellular processes. The effect of a hormone is amplified as the signaling pathway progresses. The binding of a hormone at a single receptor causes the activation of many G-proteins, which activates adenylyl cyclase. Each molecule of adenylyl cyclase then triggers the formation of many molecules of cAMP. Further amplification occurs as protein kinases, once activated by cAMP, can catalyze many reactions. In this way, a small amount of hormone can trigger the formation of a large amount of cellular product.
 |
Question 9.2
A new antagonist molecule has been discovered that binds to and blocks plasma membrane receptors. What effect will this antagonist have on testosterone, a steroid hormone? |
 |
Question 9.3
What effect will a cAMP inhibitor have on a peptide hormone-mediated signaling pathway? |
 |
Question 9.4
Name two important functions of hormone receptors. |
 |
Question 9.5
How can hormones mediate changes? |
 |
Question 9.6
List all the structures that are involved in general hormonal signaling. Once you have all the structures listed, arrange them from small to large. Next, arrange them in order of events during hormonal signaling, from first to last. |



